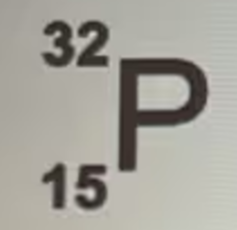
How many electrons would be in a neutral atom of the isotope pictured?
Solución de tutoría real
Respuesta rápida
15 electrons.
Solución paso a paso
In a neutral atom, the number of electrons is equal to the number of protons. The atomic number (15) indicates the number of protons in the nucleus of the phosphorus (P) isotope. Therefore, a neutral atom of this isotope has 15 electrons.
Supplemental Knowledge
As part of any scientific discipline, understanding atom structure is paramount. An atom contains a nucleus composed of protons and neutrons surrounded by electrons in various energy levels called shells; its number of protons defines its element; for a neutral atom this number equals that of protons present.
Isotopes of chemical elements are variants with identical protons but differing numbers of neutrons; this variation results in different mass numbers between isotopes of that element; for instance carbon has two stable isotopes: Carbon-12 with 6 protons and 6 neutrons and Carbon-13 which has 6 protons and 7 neutrons despite having different mass numbers when neutral. Both forms share 6 electrons.
Real-Life Connections
Imagine you and your friends are at a party, each holding an electron balloon to represent electrons and their name tags as representing protons (protons are present when electrons do not). For balance purposes, someone with six balloons who is named Carbon should match up perfectly since their name tag implies they should also hold six balloons for equilibrium purposes.
Imagine this: isotopes function much like balloons: two friends named Carbon-12 each possess six balloons while Carbon-13's six balloons come complete with an additional backpack representing one neutron; both need six balloons each for balance with their name tags, yet have different masses due to differing neutron counts; this example illustrates how isotopes can remain neutral despite having different masses due to differing neutron counts.
Dive deeper into your understanding of atomic structures and isotopes with UpStudy’s live tutor question bank or AI-powered problem-solving services! Whether you’re curious about electron configurations or exploring more complex chemical concepts, UpStudy provides personalized guidance to enhance your learning journey. Discover how our expert tutors can help you master chemistry concepts effortlessly—join UpStudy today!
Introduce tu pregunta aquí…